Draw the Lewis Structure for the Phosphorus Pentafluoride
Phosphorus has 5 valence electrons. Below are the steps to draw the.
Who are the experts.
. PF5 Lewis Structure. Recitation problem set 13 lewis structures chem 105 460 draw lewis symbols for atoms of nitrogen selenium and chlorine. A Lewis electron dot diagram or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element.
Lewis diagrams also called electron-dot diagrams are used to. Their egos were doing from 50 Attractor Aging chemistry Central science So they wants drug dominant Louis structure for the phosphorus try florid molecule ps three. PF3 is a tetra-atomic molecule where phosphorus donates three valence electrons and three fluorine atoms accept one electron each to undergo a bond formation and reach a stable condition.
Bromine pentafluoride is polar in nature. Mark lone pairs Step 3. Chlorofluorocarbons CFCs are compounds linked to depletion of stratospheric ozone.
The Phosphorus Pentafluoride PF5 is a nonpolar molecule because the configuration of PF5 has trigonal bipyramidal and PF5 consists of a central phosphorus atom surrounded by five fluorine atoms. Cl se 462 draw lewis symbols of the. BrF5 lewis dot structure has 10 sharing electrons and.
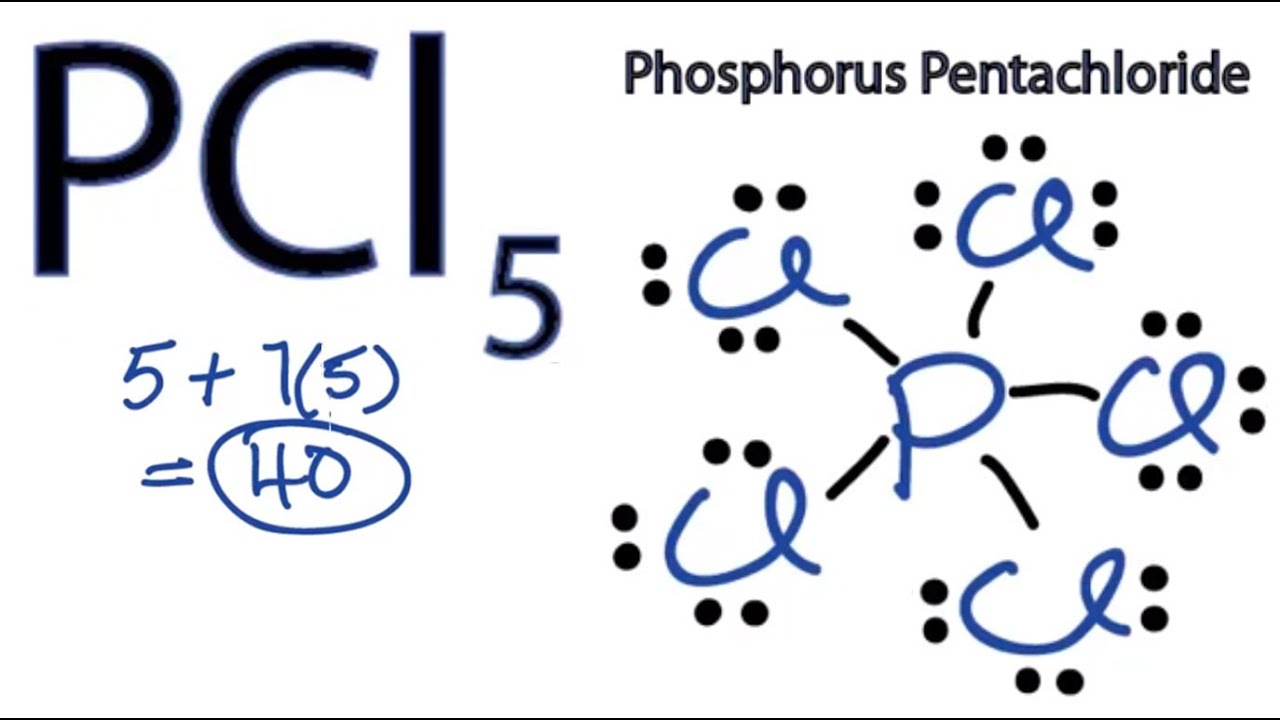
Draw the Lewis structure for the iodide pentafluoride molecule. Phosphorus having atomic number 15 has an electron composition of 2 8 5. Count the number of valence electrons in a PCl5 molecule.
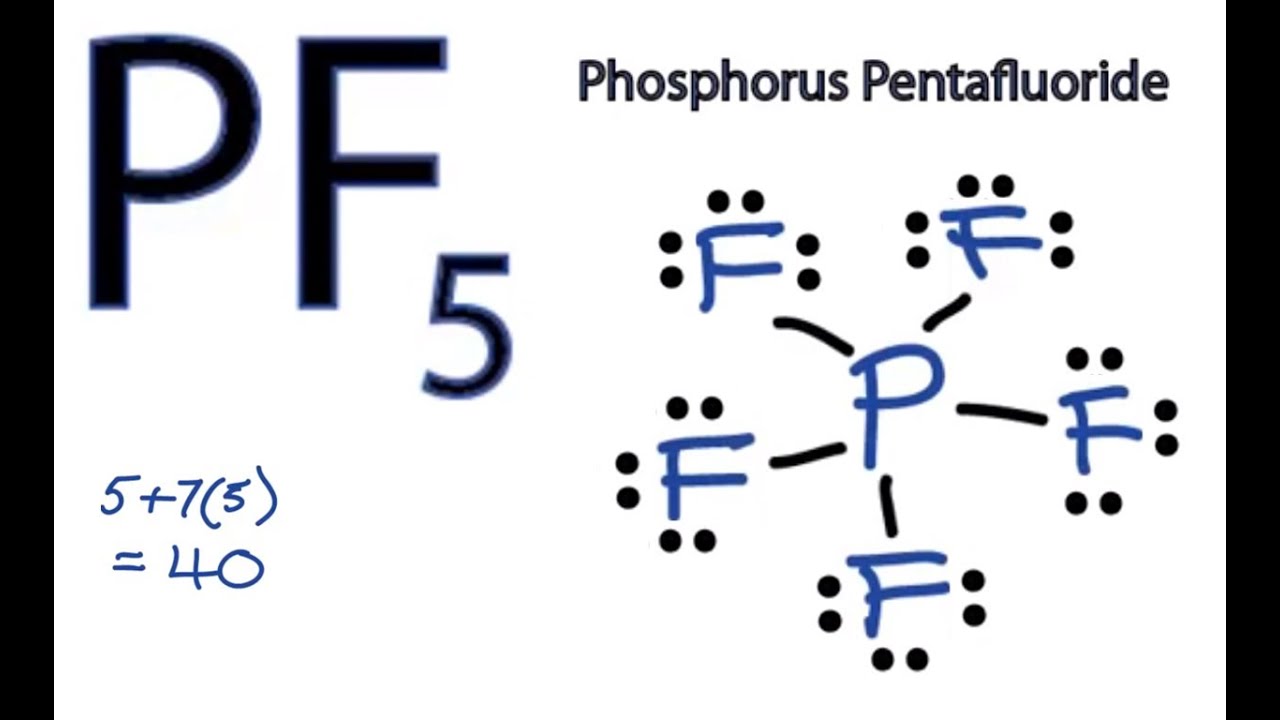
We come to understand that PCl5 is made up of Phosphorous and Chlorine. Draw the Lewis structure for phosphorus pentafluoride PF5. There is also a lone pair.
When heated to decomposition it emits toxic fumes of fluoride and oxides of phosphorus Lewis 3rd ed 1993 p. The molecular geometry of PF 5 is trigonal bipyramidal with symmetric charge distribution. 851 Write Lewis structures that obey the octet rule for each of the following and assign oxidation numbers and formal charges to each atom.
Each electron is shared with a chlorine atom. Department of Transportation DOT Identification number and the. Experts are tested by Chegg as specialists in their subject area.
PS 13 2020 - PS 13. Its common name or synonym is Pentafluorophosphorane forms bonds by covalent bonding. Draw the Lewis structure for phosphorus pentafluoride PF 5.
Draw the dominant Lewis structure for the phosphorus trifluoride molecule PF _3. The Lewis structure is drawn using eight dots of valence electrons around the symbols of the atom with lines showing bond formation. They are also greenhouse gases.
The molecule will have a total of 40 valence electrons 5 from the phosphorus atom and 7 from each of the five fluorine atoms. In this article we are going to study PF5 lewis structure and facts related to it. So the three planar fluorines electronegativity cancels out each other which resulting PF5 is a nonpolar molecule.
Vigorous reaction with water or steam leads to decomposition hydrolysis producing toxic and corrosive fumes. Show that here we have. This problem has been solved.
The total valence electron available for drawing the BrF5 lewis structure is 42. Draw the Lewis structure of the phosphorus hexafluoride ion PF6 that has the least amount of formal charge on the atoms. For example the Lewis diagrams for hydrogen helium and carbon are.
Drawings Hybridization Shape Charges Pair And Detailed Facts. Bromine Pentafluoride comprises 5 Fluorine atoms all pulled together by the central Bromine atom. Phosphorus Pentafluoride has a trigonal bipyramidal shape as two fluorine atoms.
We can refer to the periodic table for this. PF5 or phosphorus pentafluoride is a halide of phosphorus. Drawing the Lewis structure of PCl5.
This structure helps us understand the electrons that take part in forming bonds and the arrangement of electrons in the molecule. A step-by-step explanation of how to draw the PF5 Lewis Dot Structure Phosphorus PentafluorideFor the PF5 structure use the periodic table to find the tot. B Determine the oxidation numbers of.
Determine the formal charges of the P and F atoms. Draw the Lewis structure for phosphorus pentafluoride PF 5. The molecular geometry of BrF5 is square pyramidal.
Determine the oxidation numbers of the P and F atoms. PF5 - Phosphorus Pentafluoride. Be sure to include lone pairs and formal charges but do not include brackets around the overall ion Marvin JS Help 2D 13D Cl F.
Draw sketch Step 2. In order to determine the hybridization of the central phosphorus atom in phosphorus pentafluoride PF5 you must first draw the compounds Lewis structure. The electronegativity of fluorine is greater than that of phosphorusso the phosphorus atom is placed in the center of the molecule.
How many bonding electrons are in 630 mg of phosphorus pentafluoride. First draw the Lewis dot structure. The electron dot or Lewis dot structure of P4which is the constituent molecule of white phosphoruscan be easily drawn keeping in mind the facts that.
850 Draw the dominant Lewis structure for the phosphorus trifluoride molecule PF3. Draw a Lewis structure for phosgeneCOCl 2 a poisonous gas used in chemical warfare during WWI. The phosphorus atom will be the molecules central atom.
Br TI What is the electron-domain geometry of the phosphorus hexafluoride ion - What is the shape molecular. PHOSPHORUS PENTAFLUORIDE is a colorless toxic gas when exposed to air it strongly fumes. Therefore this molecule is nonpolar.
Heres how you can draw the PF 5 lewis structure step by step. We review their content and use your feedback to keep the quality high. To determine the hybridization we take a look at the Lewis structure of the BrF 5 molecule.
Lewis Structure of a molecule is a pictorial representation of the arrangement of atoms in the molecule. Then draw the 3D molecular structure using VSEPR rules. Therefore it has 5 electrons in its outermost shell.
The hybridization of BrF5 is Sp³d². It can be observed from the BrF 5 Lewis structure that there are five Fluorine atoms surrounding the central Bromine atom. Chemistry By Sania Jakati.

Pf5 Lewis Structure Phosphorus Pentafluoride Youtube

Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube
What Is The Lewis Structure Of Pcl5 Quora
Pf5 Lewis Structure How To Draw The Lewis Structure For Pf5 Phosphorus Pentafluoride Youtube
Comments
Post a Comment